Abstract
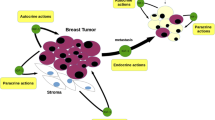
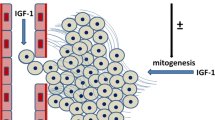
Local environmental signals regulate the growth and development of both normal and malignant breast epithelium. Members of the insulin-like growth factor (IGF) family likely influence both of these processes. The localization of IGF2 to stroma specifically surrounding malignant breast epithelium indicates that this growth factor may play a critical role in the genesis or maintenance of this transformed phenotype. Recent studies have sought to understand the mechanism by which IGF2 expressing fibroblasts are localized to the periphery of malignant breast cancer cells. In addition, the consequences of the expression of IGF-signaling components likely expand beyond their direct effects on mitogenesis. Indirect effects predominantly associated with the IGF2 receptor could also influence the invasive potential of breast tumor cells.
Similar content being viewed by others
References
Sakakura T, Nishizuka Y, Dawe CJ: Capacity of mammary fat pads of adult C3H/HeMs mice to interact morphogenetically with fetal mammary epithelium. J Natl Cancer Inst 63:733–736, 1979
Sakakura T, Sakagami Y, Nishizuka Y: Persistence of responsiveness of adult mammary gland to induction by embryonic mesenchyme. Dev Biol 72: 201–210, 1979
Streuli CH, Bailey N, Bissel MJ: Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol 115:1383–1395, 1991
Streuli CH, Bissel MJ: Expression of extracellular matrix components is regulated by substratum. J Cell Biol 110:1405–1415, 1990
Petersen OW, Rønnov-Jessen L, Howlett AR, Bissel MJ: Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA 89:9064–9068, 1992
Adams EF, Newton CJ, Braunsberg H, Shaikh N, Ghilchik M, James VHT: Effects of human breast fibroblasts on growth and 17β-estradiol dehydrogenase activity of MCF-7 cells in culture. Breast Cancer Res Treat 11:165–172, 1988
Ryan MC, Orr DJA, Horgan K: Fibroblast stimulation of breast cancer cell growth in a serum-free system. Br J Cancer 67:1268–1273, 1993
Schor AM, Rushton G, Ferguson JE, Howell A, Redford J, Schor SL: Phenotypic heterogeneity in breast fibroblasts: functional anomaly in fibroblasts from histologically normal tissue adjacent to carcinoma. Int J Cancer 59:25–32, 1994
Brouty-Boye D, Mainguene C, Magnien V, Israel L, Beaupain R: Fibroblast-mediated differentiation in human breast carcinoma cells (MCF-7) grown as nodules in vitro. Int J Cancer 56:731–735, 1994
Azzarone B, Marcel M, Billard C, Scemama P, Chaponnier C, Macieira-Coelho A: Abnormal properties of skin fibroblasts from patients with breast cancer. Int J Cancer 33:759–764, 1984
Durning P, Schor SL, Sellwood RAS: Fibroblasts from patients with breast cancer show abnormal migratory behaviour in vitro. Lancet ii:890–892, 1984
Schor SL, Haggie J, Durning P, Howell A, Sellwood RAS, Crowther D: The occurrence of a foctal fibroblast phenotype in breast cancer. Int J Cancer 37:831–836, 1986
Haggie J, Schor SL, Howell A. Birch JM, Sellwood RAS: Fibroblasts from relatives of hereditary breast cancer display foetal-like behaviour in vitro. Lancet i:1455–1457, 1987
Jones JI, Clemmons DR: Insulin-like growth factors and their binding proteins: biological actions. Endocrine Reviews 16:3–34, 1995
Nishimoto I, Murayama Y, Katada T, Um M, Ogata E: Possible direct linkage of insulin-like growth factor-II receptor with guanine nucleotide-binding proteins. J Biol Chem 264:14029–14038, 1989
Korner C, Nurnberg B, Uhde M, Braulke T: Mamose 6-phosphate/insulin-like growth factor-II receptor fails to interact with G-proteins. J Biol Chem 270:287–295, 1995
Filson AJ, Louvi A, Efstratiadis A, Robertson EJ: Rescue of the T-associated maternal effect in mice carrying null mutations in Igf-2 and M6P/IGF2R, two reciprocally imprinted genes. Development 118:731–736, 1993
Ellis MJC, Leav BA, Yang Z, Rasmussen A, Pearce A, Zwibel JA, Lippman ME, Cullen KJ: Affinity for the insulin-like growth factor-II (IGF-II) receptor inhibits autocrine IGF-II activity in MCF-7 breast cancer cells. Mol Endocrinol 10:286–297, 1996
Hankins GR, De Souza AT, Bentley RC, Patel MR, Marks JR, Iglehart JD, Jirtle RL: M6P/IGF2 receptor: a candidate breast tumor suppressor gene. Oncogene 12:2003–2009, 1996
Bondy CA, Werner AH, Roberts CT Jr, LeRoith D: Cellular pattern of insulin-like growth factor-1 (IGF1) and type-1 IGF receptor gene expression in early organogenesis: comparison with IGF2 gene expression. Mol Endocrinol 4:1386–1398, 1990
Liu J-P, Baker J, Perkins AS, Robertson EJ, Efstratiadis A: Mice carrying null mutations of the genes encoding insulin-like growth factor 1 (IGF1) and Type I IGF receptor (IGF1r). Cell 75:59–72, 1993
DeChiara TM, Efstratiadis A, Robertson EJ: A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345:78–80, 1990
Voss JW, Rosenfeld MG: Anterior pituitary development: short tales from dwarf mice. Cell 70:527–530, 1992
Woods KA, Camacho-Hübner C, Savage MO, Clark AJL: Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335:1363–1367, 1996
Baserga R, Rubin R: Cell cycle and growth control. Critical Rev Eukatyotic Gene Expr 3:47–61, 1993
Resnicoff M, Burgaud J-L, Rotman HL, Abraham D, Baserga R: Correlation between apoptosis, tumorigenesis, and levels of insulin-like growth factor I receptors. Cancer Res 55:3739–3741, 1995
Cullen KJ, Yee D, Rosen N: Insulin-like growth factors in human malignancy. Cancer Invest 9:443–454, 1991
Singer C. J Clin Endo Metab, in press, 1997
Mathews LS, Hammer RE, Behringer RR, D'Ercole AJ, Bell GI, Brinster RL, Palmiter RD: Growth enhancement of transgenic mice expressing human insulin-like growth factor 1. Endocrinology 123:2827–2833, 1988
Rogler CE, Yang D, Rossetti L, Donohoe J, Alt E, Chang CJ, Rosenfeld R, Neely K, Hintz R: Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J Biol Chem 269:13779–13784, 1994
Bates P, Fisher R, Ward A, Richardson L, Hill J, Graham CF: Mammary cancer in transgenic mice expressing insulin-like growth factor II (IGF-II). Br J Cancer 72:1189–1193, 1995
Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, Rosen N: Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res 50:48–53, 1990
Yee D, Paik S, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, Lippman ME, Rosen N: Analysis of insulin-like growth factor I gene expression in malignancy: evidence for a paracrine role in human breast cancer. Mol Endocrinol 3:509–517, 1989
Yee D, Cullen KJ, Paik S, Perdue JF, Hampton B, Schwartz A, Lippman ME, Rosen N: Insulin-like growth factor II mRNA expression in human breast cancer. Cancer Res 48:6691–6696, 1988
Toropainen E, Lipponen P, Syrjänen K: Expression of insulin-like growth factor I (IGF-I) in female breast cancer as related to established prognostic factors and long-term prognosis. Eur J Cancer 31A: 1443–1448, 1995
Toropainen EM, Lipponen PK, Syrjänen KJ: Expression of insulin-like growth factor II in female breast cancer as related to established prognostic factors and long-term prognosis. Anticancer Res 15: 2669–2674, 1995
Pekonen F, Partanen S, Makinen T, Rutanen EM: Receptors for epidermal growth factor and insulin-like growth factor I and their relation to steroid receptors in human breast cancer. Cancer Res 48: 1343–1347, 1988
Paik S: Expression of IGF-I and IGF-II mRNA in breast tissue. Breast Cancer Res Treat 22:31–38, 1992
Giani C, Cullen KJ, Campani D, Rasmussen A: IGF-II mRNA and protein are expressed in the stroma of invasive breast cancers: an in situ hybridization and immunohistochemistry study. Breast Cancer Res Treat 41:43–50, 1996
Cullen KJ, Smith HS, Hill S, Paik S, Rosen N, Lippman ME: Growth factor mRNA expression by human breast fibroblasts from benign and malignant lesions. Cancer Res 51:4978–4985, 1991
Singer C, Rasmussen A, Smith HS, Lippman ME, Cullen KJ: Malignant breast epithelium induces insulin-like growth factor II expression in breast stroma. Evidence for paracrine function. Cancer Res 55:2448–2454, 1995
Manni A, Badger B, Wei L, Zaenglein A, Grove R, Khin S, Heitjan D, Shimasaki S, Ling N: Hormonal regulation of insulin-like growth factor II and insulin-like growth factor binding protein expression by breast cancer cells in vivo: evidence for stromal epithelial interactions. Cancer Res 54:2934–2942, 1994
Ruan W, Newman CB, Kleinberg DL: Intact and amino-terminally shortened forms of insulin-like growth factor I induce mammary gland differentiation and development. Proc Natl Acad Sci USA 89:10872–10876, 1992
Zhuang Z, Merino MJ, Chuaqui R, Liotta LA, Emmert-Buck MR: Identical allelic loss on chromosome 11q13 in microdissected in situ and invasive human breast cancer. Cancer Res 55:467–471, 1995
Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery J-P, Koteliansky VE: Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA 90:999–1003, 1993
Rønnov-Jessen L, Petersen OW, Koteliansky VE, Bissel MJ: The origin of myofibroblasts in breast cancer. J Clin Invest 95:859–873, 1995
White MF, Kahn CR: The insulin signaling system. J Biol Chem 269:1–4, 1994
Singleton JR, Dixit VM, Feldman EL: Type I insulin-like growth factor receptor activation regulates apoptotic proteins. J Biol Chem 271: 31791–31794, 1996
O'Conner R, Kauffmann-Zeh A, Liu Y, Lehar S, Evan GI, Baserga R, Blattler WA: Identification of domains of the insulin-like growth factor I receptor that are required for protection from apoptosis. Mol Cell Biol 17:427–435, 1997
Pollak MN, Huynh HT, Lefebvre SP: Tamoxifen reduces serum insulin-like growth factor I (IGF-I). Breast Cancer Res Treat 22:91–100, 1992
Ellis MJ, Singer C, Hornby A, Rasmussen A, Cullen KJ: Insulin-like growth factor stromal-epithelial interactions in human breast cancer. Breast Cancer Res Treat 31:153–165, 1994
Cullen KJ, Lippman ME, Chow D, Hill S, Rosen N, Zwibel JA: Insulin-like growth factor-II overexpression in MCF-7 cells induces phenotypic changes associated with malignant progression. Mol Endocrinol 6:91–100, 1992
Lee AV, Darbre P, King RJB: Processing of insulin-like growth factor-II (IGF-II) by human breast cancer cells. Mol Cell Endocrinol 99:211–220, 1994
Bonneterre J, Peyrat JP, Beuscart R, Demaille A: Prognostic significance of insulin-like growth factor 1 receptors in human breast cancer. Cancer Res 50:6931–6935, 1990
Stewart AJ, Johnson MD, May FEB, Westley BR: Role of IGFs and the type I IGF receptor in the estrogen stimulated proliferation of human breast cancer cells. J Biol Chem 265:21172–21178, 1990
Mathieu M, Rochefort H, Barenton B, Prebois C, Vignon F: Interactions of cathepsin-D and insulin-like growth factor-II (IGF-II) on the IGF-II/mannose-6-phosphate receptor in human breast cancer cells and possible consequences on mitogenic activity of IGF-II. Mol Endocrinol 4:1327–1335, 1990
Osborne CK, Clemmons DR, Arteaga CL: Regulation of breast cancer growth by insulin-like growth factors. J Steroid Biochem Molec Biol 37:805–809, 1992
Pekonen F, Nyman T, Ilvesmäki V, Partanen S: Insulin-like growth factor binding proteins in human breast cancer tissue. Cancer Res 52:5204–5207, 1992
Rocha RL, Hilsenbeck SG, Jackson JG, Lee AV, Figueroa JA, Yee D: Correlation of insulin-like growth factor-binding protein-3 messenger RNA with protein expression in primary breast cancer tissues: detection of higher levels in tumors with poor prognostic features. J Natl Cancer Inst 88:601–606, 1996
van der Berg HW, Claffie D, Boylan M, McKillen J, Lynch M, McKibben B: Expression of receptors for epidermal growth factor and insulin-like growth factor I by ZR-75-1 human breast cancer cell variants is inversely related to the effect of steroid hormone on insulin-like growth factor I expression. Br J Cancer 73:477–481, 1996
Helle SI, Anker, GB, Tally M, Hall K, Lonning PE: Influence of droloxifene on plasma levels of insulin-like growth factor (IGF)-I, pro-IGF-IIE, insulin-like growth factor binding protein (IGFBP)-1 and IGFBP-3 in breast cancer patients. J Steroid Biochem Mol Biol 57:167–171, 1996
Brünner N, Yee D, Kern FG, Spang-Thomsen M, Lippman ME, Cullen KJ: Effect of endocrine therapy on growth of T61 human breast cancer xenografts is directly correlated to a specific down-regulation of insulin-like growth factor II (IGF-II). Eur J Cancer 29A:562–569, 1993
Arteaga CL, Osborne CK: Growth inhibition of human breast cancer cells in vitro with an antibody against the type I somatomedin receptor. Cancer Res 49:6237–6241, 1989
Arteaga CL, Kitten LJ, Coronado EB, Jacobs S, Kull FC, Allred DC, Osborne CK: Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest 84:1418–1423, 1989
Kenney NJ, Dickson RB: Growth and sex steroid interactions in breast cancer. J Mammary Gland Biol Neoplasia 1:189–198, 1996
Daws MR, Westley BR, May FEB: Paradoxical effects of overexpression of the type I insulin-like growth factor (IGF) receptor on the responsiveness of human breast cancer cells to IGFs and estradiol. Endocrinology 137:1177–1186, 1996
Tandon AK, Clark GM, Chamness GC, Chirgwin JM, McGuire WL: Cathepsin D and prognosis in breast cancer. N Engl J Med 322:297–302, 1990
Spyratos F, Maudelonde T, Brouillet JP, Brunet M, Defrenne A, Andrieu C, Hacene K, Desplaces A, Rouessé J, Rochefort H: Cathepsin D: an independent prognostic factor for metastasis of breast cancer. Lancet ii:1115–1118, 1989
Hoeflich A, Wolf E, Braulke T, Koepf G, Kessler U, Brem G, Rascher W, Blum WF, Liess W: Does the overexpression of pro-insulin-like growth factor-II in transfected human embryonic kidney fibroblasts increase the secretion of lysosomal enzymes? Eur J Biochem 232:172–178, 1995
De Leon DD, Terry C, Asmerom Y, Nissley P: Insulin-like growth factor II modulates the routing of cathepsin D in MCF-7 breast cancer cells. Endocrinology 137:1851–1859, 1996
Briozzo P, Morisset M, Capony F, Rougeot C, Rochefort H: In vitro degradation of extracellular matrix with Mr 52,000 cathepsin D secreted by breast cancer cells. Cancer Res 48:3688–3692, 1988
Henry JA, McCarthy AL, Angus B, Westley BR, May FEB, Nicholson S, Cairns J, Harris AL, Horne CHW: Prognostic significance of the estrogen-regulated protein, cathepsin D, in breast cancer. An immunohistochemical study. Cancer 65:265–271, 1990
Johnson MD, Torri JA, Lippman ME, Dickson RB: The role of cathepsin D in the invasiveness of human breast cancer. Cancer Res 53:873–877, 1993
Mignatti P, Rifkin DB: Biology and biochemistry of proteinases in tumor invasion. Physiol Rev 73:161–195, 1993
Dennis PA, Rifken DB: Cellular activation of latent transforming growth factor β requires binding to the cation-independent mannose 6-phosphate/insulin like growth factor type II receptor. Proc Natl Acad Sci USA 88:580–584, 1991
Dalal BI, Keown PA, Greenberg AH: Immunocytochemical localization of secreted transforming growth factor-beta 1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am J Pathol 143:381–389, 1993
Holst-Hansen C, Johannessen B, Høyer-Hansen G, Rømer J, Ellis V, Brünner N: Urokinase-type plasminogen activation in three human breast cancer cell lines correlates with their in vitro invasiveness. Clin Exp Metastasis 14:297–307, 1996
Arnoletti JP, Albo D, Granick MS, Solomon MP, Castiglioni A, Rothman VL, Tuszynski GP: Thrombo spondin and transforming growth factor-beta 1 increase expression of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 in human MDA-MB-231 breast cancer cells. Cancer 76:998–1005, 1995
Falcone DJ, McCaffrey TA, Haimovitz-Friedman A, Garcia M: Transforming growth factor-β1 stimulates macrophage urokinase expression and release of matrix-bound basic fibroblast growth factor. J Cell Physiol 155:595–605, 1993
Minniti CP, Kohn EC, Grubb JH, Sly WS, Oh Y, Mueller HL, Rosenfeld RG, Helman LJ: The insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor mediates IGF-II-induced motility in human rhabdomyosarcoma cells. J Biol Chem 267:9000–9004, 1992
Nakao-Hayashi J, Ito H, Kanayasu T, Morita I, Muiota S-I: Stimulatory effects of insulin and insulin-like growth factor 1 on migration and tube formation by vascular endothelial cells. Atherosclerosis 92:141–149, 1992
Klemke RL, Yebra M, Bayna EM, Cheresh DA: Receptor tyrosine kinase signaling required for integrin alpha V beta 5-directed cell motility but not adhesion on vitronectin. J Cell Biol 127:859–866, 1994
Jones JI, Prevette T, Gockerman A, Clemmons DR: Ligand occupancy of the αVβ3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor I. Proc Natl Acad Sci USA 93:2482–2487, 1996
Doerr ME, Jones JI: The roles of integrins and extracellular matrix proteins in the insulin-like growth factor I-stimulated chemotaxis of human breast cancer cells. J Biol Chem 271:2443–2447, 1996
Stracke ML, Engel JD, Wilson LW, Rechler MM, Liotta LA, Schiffmann E: The type I insulin-like growth factor receptor is a motility receptor in human melanoma cells. J Biol Chem 264:21544–21549, 1989
Grant MB, Guay C, Marsh R: Insulin-like growth factor I stimulates proliferation, migration, and plasminogen activator release by human retinal pigment epithelial cells. Curr Eye Res 9:323–335, 1990
Volpert O, Jackson D, Bouck N, Linzer DIH: The insulin-like growth factor II/mannose 6-phosphate receptor is required for proliferin-induced angiogenesis. Endocrinology 137:3871–3876, 1996
Beukers MW, Oh Y, Zhang H, Ling N, Rosenfeld R: [Leu 27] insulin-like growth factor II is highly selective for the type-II IGF receptor in binding, crosslinking and thymidine incorporation experiments. Endocrinology 128:1201–1203, 1991
Roesel JF, Nanney LB: Assessment of differential cytokine effects on angiogenesis using an in vivo model of cutaneous wound repair. J Surg Res 58:449–459, 1995
Grant MB, Mames RN, Fitzgerald C, Ellis EA, Aboufriekha M, Guy J: Insulin-like growth factor I acts as an angiogenic agent in rabbit cornea and retina: comparative studies with basic fibroblast growth factor. Diabetologia 36:282–291, 1993
Lefaucheur J-P, Gjata B, Lafont H, Sebille A: Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-β1. J Neuroimmunology 70:37–41, 1996
Pepper MS, Vassalli J-D, Orci L, Montesano R: Biphasic effect of transforming growth factor-β1 on in vitro angiogenesis. Exp Cell Res 204:356–363, 1993
Briozzo P, Badet J, Capony F, Pieri I, Montcourrier P, Barritault D, Rochefort H: MCF-7 mammary cancer cells respond to bFGF and internalize it following its release from extracellular matrix: a permissive role of cathepsin D. Exp Cell Res 194: 252–259, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rasmussen, A.A., Cullen, K.J. Paracrine/autocrine regulation of breast cancer by the insulin-like growth factors. Breast Cancer Res Treat 47, 219–233 (1998). https://doi.org/10.1023/A:1005903000777
Issue Date:
DOI: https://doi.org/10.1023/A:1005903000777