Abstract
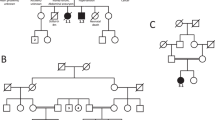
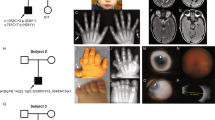
Mutation or deletion of the PAX6 gene underlies many cases of aniridia. Three lines of evidence now converge to implicate PAX6 more widely in anterior segment malformations including Peters' anomaly. First, a child with Peters' anomaly is deleted for one copy of PAX6. Second, affected members of a family with dominantly inherited anterior segment malformations, including Peters' anomaly are heterozygous for an R26G mutation in the PAX6 paired box. Third, a proportion of Sey/+ Smalleye mice, heterozygous for a nonsense mutation in murine Pax–6, have an ocular phenotype resembling Peters' anomaly. We therefore propose that a variety of anterior segment anomalies may be associated with PAX6 mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Peters, A. Uber angeboren Defektbildung der Descemetschen Membran. Klin. Mbl. Augenheilk. 44, 27–40 (1906).
Stone, D.L., Kenyon, K.R., Green, W.R. & Ryan, S.J. Congenital central corneal leukoma (Peters anomaly). Am. J. Ophthalmol. 81, 173–193 (1976).
Holmstrom, G.E., Reardon, W.P., Baraitser, M., Elston, J.S. & Taylor, D.S., Heterogeneity In dominant anterior segment malformations. Br. J. Ophthalmol. 75, 591–597 (1991).
Hittner, H.M., Kretzer, F.L., Arrtoszyk, J.H., Ferrell, R.E. & Mehta, R.S. Variable expressivity of autosomal dominant anterior segment mesenchymal dysgenesis in six generations. Am. J. Ophthalmol. 93, 57–70 (1982).
Bopp, D., Burri, M., Baumgartner, S., Frigerio, G. & Noll, M. Conservation of a large protein domain in the segmentation gene paired and functionally related genes of Drosophila. Cell 47, 1033–1040 (1986).
Walther, C. et al. Pax: a murine multigene family of paired box-containing genes. Genomics 11, 424–434 (1991).
Chalepakis, G., Tremblay, P. & Gruss, P. Pax genes, mutants and molecular function. J. Cell Science (suppl.) 16, 61–67 (1992).
Ton, C.C.T. et al. Positional cloning and characterization of a paired box-and homeobox-containing gene from the aniridia region. Cell 67, 1059–1074 (1991).
Walther, C. & Gruss, P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 113, 1435–1449 (1991).
Jordan, T. et al. The human PAX6 gene is mutated in two patients with aniridia. Nature Genet. 1, 328–332 (1992).
Glaser, T., Walton, D.S. & Maas, R.L. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nature Genet. 2, 232–239 (1992).
Hanson, I.M. et al. PAX6 mutations in aniridia. Hum. molec. Genet. 2, 915–920 (1993).
Hill, R.E. et al. Mouse Small eye results from mutations in a paired-like homeobox-containing gene. Nature 354, 522–525 (1991).
Fantes, J.A. et al. Non-radioactive in situ hybridization for the rapid analysis of submicrosoopic deletions at the WAGR locus. Am. J. hum. Genet. 51, 1286–1294 (1992).
Martin, P. et al. Characterization of a paired box-and homeobox-containing quail gene (Pax-QNR) expressed in the neuroretlna. Oncogene 7, 1721–1728 (1992).
Krauss, S. et al. Zebrafish pax[zf-a]: a paired box-containing gene expressed in the neural tube. EMBO J. 10, 3609–3619 (1991).
Burri, M., Tromvoukis, Y., Bopp, D., Frigerio, G. & Noll, M. Conservation of the paired domain in metazoans and its structure in three isolated human genes. EMBO J. 8, 1183–1190 (1989).
Treisman, J., Harris, E. & Desplan, C. The paired box encodes a second DNA-bindlng domain in the Paired homeo domain protein. Genes Dev. 5, 594–604 (1991).
Hoth, C.F. et al. Mutations in the paired domain of the human PAX3 gene cause Klein-Waardenburg syndrome (WS-III) as well as Waardsnburg syndrome type I (WS-I). Am. J. hum. Genet. 52, 455–462 (1993).
Glaser, T., Lane, J. & Housman, D. A mouse model of the aniridia-Wilms' tumor deletion syndrome. Science 250, 823–827 (1990).
Green, M.C. Catalog of mutant genes and polymorphic loci. In Genetic variants and strains of the laboratory mouse (2nd edn) (eds M. F. Lyon & A. G. Searie) 12–403 (Oxford University Press, Oxford, (1989).
Clayton, R.M. Developmental genetics of the lens. In The ocular lens (ed. H. Maisel) 61–92 (Marcel Dekker Inc, New York, (1985).
Hogan, B.L.M. et al. Small eyes (Sey):a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J. Embryol. exp. Morph. 97, 95–110 (1986).
Hogan, B.L.M., Hirst, E.M.A., Horsburgh, G. & Hetherington, C.M. Small eye (Sey): a mouse model for the genetic analysis of craniofacial abnormalities. Development (suppl.) 103, 115–119 (1988).
van Heyningen. V. et al. Role for the Wilms tumor gene in genital development? Proc. natn. Acad. Sci. U.S.A. 87, 5383–5383 (1990).
Beauchamp, G.R. Anterior segment dysgenesis keratolenticular adhesion and aniridia. J. paed. Ophthalmol. Strabismus 17, 55–58 (1978).
Jotterand, V. et al. 11p13 deletion, Wilms' tumour, and aniridia: unusual genetic, non-ocular and ocular features of three cases. Br. J. Ophthalmol. 74, 568–570 (1990).
Eiferman, R.A. Association of Wilms tumour with Peters anomaly. Ann. of Ophthalmology 16, 933–934 (1984).
Waring, G.O., Rodrigues, M.M. & Laibson, P.R. Anterior chamber cleavage syndrome. A stepladder classification. Surv. Ophthalmol. 20, 3–27 (1975).
Hittner, H.M., Riccardi, V.M., Ferrell, R.E., Borda, R.R. & Justice, J. Variable expressivity in autosomal dominant aniridia by clinical, electrophysiologic and angiographic criteria. Am. J. Ophthalmol. 89, 531–539 (1980).
Beattie, P.H. A consideration of aniridia with a pedigree. Br. J. Ophthalmol. 31, 649–676 (1947).
Kenyon, K.R. Mesenchymal dysgenesis in Peters anomaly, sclerocornea and congenital endothelial dystrophy. Exp. Eye Res. 21, 125–142 (1975).
Murray, J.C. et al. Linkage of Rieger syndrome to the region of the epidermal growth factor gene on chromosome 4. Nature Genet. 2, 46–49 (1992).
Heon, E. et al. Peters anomaly. The spectrum of associated ocular and systemic malformations. Ophthal. Paed. 13, 137–143 (1992).
Walter, M.A., Goodfellow, P.N. Disease and development. Nature 355, 590–591 (1992).
Asher, J.H., Morell, R. & Friedman, T.B. Waardenburg Syndrome (WS): the analysis of a single family with a WSI mutation chowlng linkage to RFLP markers on human chromosome 2q. Am. J. hum. Genet. 48, 43–52 (1991).
Tassabehji, M. et al. Mutations in the PAX3 gene causing Waardenburg syndrome type 1 and type 2. Nature Genet. 3, 26–29 (1993).
Hill, R.E. & van Heyningen, V. Mouse mutations and human disorders are Paired. Trends Genet. 8, 119–120 (1992).
Lichter, P. et al. High-resolution mapping of human chromosomes 11 by in situ hybridization with cosmid clones. Science 247, 64–69 (1990).
Pinkel, D., Straume, T. & Gray, J.W. Cytogenetic analysis using quantitative, high sensitivity, fluorescence hybridization. Proc. natn. Acad. Sci. U.S.A. 83, 2934–2938 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hanson, I., Fletcher, J., Jordan, T. et al. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat Genet 6, 168–173 (1994). https://doi.org/10.1038/ng0294-168
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0294-168
This article is cited by
-
Impaired DNA-binding affinity of novel PAX6 mutations
Scientific Reports (2020)
-
Two sisters with microphthalmia and anterior segment dysgenesis secondary to a PAX6 pathogenic variant with clinically healthy parents: a case of gonadal mosaicism?
Japanese Journal of Ophthalmology (2020)
-
CPAMD8 loss-of-function underlies non-dominant congenital glaucoma with variable anterior segment dysgenesis and abnormal extracellular matrix
Human Genetics (2020)
-
Phenotype–genotype correlations and emerging pathways in ocular anterior segment dysgenesis
Human Genetics (2019)
-
Through the looking glass: eye anomalies in the age of molecular science
Human Genetics (2019)