Article Text
Abstract
BACKGROUND Tumour necrosis factor (TNF) is a predominant cytokine produced in the gastric mucosa of patients with Helicobacter pyloriinfection. TNF induces apoptosis in a variety of cells. The soluble TNF receptors (sTNF-Rs) can be divided into sTNF-RI and sTNF-RII, both of which inhibit TNF activity. However, their precise mechanisms remain unclear.
AIM To investigate the role of sTNF-Rs in H pylori infection.
METHODS In 40 patients, production of TNF and sTNF-Rs in gastric mucosa was measured using biopsy specimens. In addition, in gastric epithelial cells, sTNF-R release in response to TNF and the protective effect of sTNF-Rs against the cytotoxic and apoptotic activities of TNF were examined.
RESULTS TNF and sTNF-R expression was significantly higher in H pylori positive than H pylorinegative patients. TNF dose-dependently induced sTNF-RI release from gastric epithelial cells. sTNF-RII was also released from the cells. TNF decreased cell viability, but the effect was very small. A combination of anti-sTNF-RI and anti-sTNF-RII monoclonal antibodies significantly increased TNF induced cytotoxicity and apoptosis of gastric epithelial cells.
CONCLUSIONS These results show that sTNF-Rs are actively produced inH pylori infected gastric mucosa. sTNF-Rs appear to protect gastric epithelial cells from TNF induced apoptosis in H pylori infection.
- Helicobacter pylori
- tumour necrosis factor
- soluble TNF receptors
- apoptosis
- gastric mucosa
- stomach
Abbreviations used in this paper
- TNF
- tumour necrosis factor
- sTNF-R
- soluble TNF receptor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PCR
- polymerase chain reaction
Statistics from Altmetric.com
Helicobacter pylori infection is considered to be a risk factor for gastric cancer,1-4 but the mechanisms underlying its carcinogenic potential remain unclear. Recent studies have indicated an increase in gastric epithelial cell apoptosis in mucosal biopsy specimens from patients withH pylori associated gastritis,5-8 and it is possible that excessive apoptosis caused by H pylori infection may favour carcinogenesis.5-8 A variety of stimuli can induce apoptosis, such as tumour necrosis factor (TNF).9-11Several studies have suggested that TNF is produced byH pylori infection12-14 and is closely related to epithelial injury.11
TNF exerts pleiotropic effects by linking two high affinity TNF receptors (TNF-Rs) of 55 and 75 kDa on a variety of cells.15 Soluble forms of the human 55 kDa TNF-R (sTNF-RI) and the 75 kDa receptor (sTNF-RII) appear to be released from the cell surface by proteolytic cleavage of the extracellular domains of these membrane associated receptors.16 ,17 Soluble receptors inhibit TNF activity by binding to TNF thus preventing its binding to membrane associated TNF-Rs.18 ,19 Recently, urine and serum from healthy, febrile, and HIV infected patients have been found to contain sTNF-Rs,20-22 but there have been no reports on sTNF-R expression in the stomach. The aims of this study were to investigate gastric TNF expression and sTNF-R release in patients withH pylori infection, and to clarify the protective effect of sTNF-Rs against the cytotoxic and apoptotic activity of TNF.
Methods
PATIENTS
Forty asymptomatic individuals (36 men and four women aged 29–70 years; mean age, 51.9 years) who were having a routine health examination were studied. None of them had recently taken antibiotics, non-steroidal anti-inflammatory drugs, or bismuth compounds. The project was approved by the Second Department of Internal Medicine at Nagoya University School of Medicine and the Aichi Prefectural Center for Health Care. Informed consent to the study was obtained from each subject, and upper gastrointestinal endoscopy was performed. Four biopsy specimens were obtained from the greater curvature at the gastric antrum and one was obtained from the gastric body. A biopsy specimen from the antrum and one from the body were used for the rapid urease test (CLO test).23 Another specimen from the gastric antrum was used for histological examination. The presence and severity of gastritis were graded in accordance with the Sydney system24; inflammation, activity, atrophy, and intestinal metaplasia were scored from 0 to 3 on haematoxylin and eosin stained sections (0, none; 1, mild; 2, moderate; 3, severe). The two residual specimens were used for tissue culture. Infection withH pylori was defined by histological or CLO test positivity.
TISSUE CULTURE
Biopsy specimens for culture were immediately placed in 1 ml RPMI 1640 medium. After six hours, the medium was changed and further incubation was carried out in the presence of 5% CO2 at 37°C for 24 hours. At the end of the incubation, culture supernatants were collected and stored at −80°C until assayed. The specimens were homogenised in 0.5 ml 1 M NaOH at 100°C for 10 minutes and neutralised with the same volume of 1 M HCl. Aliquots of the homogenate were assayed for total protein by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, California, USA).
MEASUREMENT OF TNF, sTNF-RI, AND sTNF-RII IN TISSUE CULTURE
Concentrations of TNF, sTNF-RI, and sTNF-RII in tissue culture supernatants were determined by enzyme linked immunosorbent assay (ELISA) (Quantikine; R&D Systems, Minneapolis, Minnesota, USA). TNF and sTNF-R concentrations were expressed as pg/mg protein.
CELL LINE
A human gastric epithelial cell line MKN45 was provided by the Japanese Cancer Research Resources Bank. Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin (105U/l), and streptomycin (100 mg/l). In some comparative studies, a human gastric epithelial cell line, KATO III, was also used. Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented as for the MKN45 cells.
MEASUREMENT OF TNF, sTNF-RI, AND sTNF-RII IN CELL CULTURE
Concentrations of sTNF-RI and sTNF-RII in cell culture supernatants were determined by the same method as the tissue culture. Briefly, MKN45 cells (2 × 105/well) were plated into 12-well culture plates and cultured in 1 ml complete medium with or without various concentrations of TNF (1–100 ng/ml) for 24 hours at 37°C. KATO III cells were also plated by the same method and cultured with or without TNF (100 ng/ml).
3-(4,5-DIMETHYLTHIAZOL-2-YL)-2,5-DIPHENYL TETRAZOLIUM BROMIDE (MTT) ASSAY
The effect of human TNF (Bachem Fine Chemicals Inc, Torrance, California, USA) on MKN45 cell viability was evaluated by the MTT reduction assay (Chemicon International Inc, Temecula, California, USA). Briefly, MKN45 cells (2 × 103/well) were plated into 96-well culture plates and cultured in 100 μl complete medium with or without various concentrations of TNF (1–100 ng/ml). After 24–96 hours, MTT (0.5 mg/ml) was added and the reaction was allowed to proceed for four hours at 37°C, after which intracellular formazan was extracted with 100 μl/well acid propan-2-ol. The absorbance of each well was measured with a dual wavelength automated plate reader (MTP-100; Corona Electric Co, Ibaragi, Japan) at 570/650 nm.25
This method was also used to investigate effects of anti-sTNF-R monoclonal antibodies (R&D Systems) on cell viability. MKN45 cells (2 × 103/well) were cultured in the presence of anti-sTNF-RI and/or anti-sTNF-RII monoclonal antibodies for three hours, and further incubated with TNF (100 ng/ml) for 21 hours. The conditions were TNF + anti-sTNF-RI monoclonal antibody (40 μg/ml), TNF + anti-sTNF-RII monoclonal antibody (40 μg/ml), TNF + anti-sTNF-RI monoclonal antibody (10 μg/ml) + anti-sTNF-RII monoclonal antibody (10 μg/ml) and TNF + anti-sTNF-RI monoclonal antibody (20 μg/ml) + anti-sTNF-RII monoclonal antibody (20 μg/ml). The medium was changed daily until the cells had been incubated for 96 hours.
TARGET OF ANTI-sTNF-R MONOCLONAL ANTIBODIES
To investigate whether anti-sTNF-Rs monoclonal antibodies used in this study bind to shed TNF-Rs rather than the membrane bound ones, the following studies were carried out. We extracted proteins from cell lysate of untreated MKN45 cells and cell culture supernatant treated with 100 ng/ml TNF and performed western blot analysis. The cell culture supernatant was concentrated using a Microcon microconcentrator with 50 kDa cut off membrane. Samples (5 μg/lane) were electrophoresed and transferred to the membrane. After being blocked, the membrane was incubated with a mouse monoclonal antibody against human sTNF-RI and sTNF-RII. The membrane was washed and then incubated with peroxidase conjugated goat anti-mouse IgG.
DNA ELECTROPHORESIS
Genomic DNA was extracted from untreated and treated (TNF (100 ng/ml) and TNF (100 ng/ml) + anti-sTNF-RI monoclonal antibody (20 μg/ml) + anti-sTNF-RII monoclonal antibody (20 μg/ml)) cells (1 × 105/well) after 96 hours. Briefly, cells were harvested and lysed in 10 mM Tris/HCl (pH 8.0) containing 10 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulphate and 100 μg/ml proteinase K for one hour at 55°C, and then gently mixed for 16 hours at 37°C. DNA was then extracted twice with phenol and phenol/chloroform, and recovered in the aqueous phase. DNA was precipitated in ethanol, spun for 15 min at 15 000 g, and resuspended in TE buffer (10 mM Tris/HCl, pH 8.0, 1 mM EDTA) containing 100 μg/ml RNase for at least one hour at 37°C. Electrophoretic separation of DNA (20 μg per lane) was carried out on 2% agarose gel.
FLOW CYTOMETRIC ANALYSIS
To detect apoptosis, flow cytometric analysis was performed as previously described.26-28 Untreated and treated (TNF (100 ng/ml) and TNF (100 ng/ml) + anti-sTNF-RI monoclonal antibody (20 μg/ml) + anti-STNF-RII monoclonal antibody (20 μg/ml)) MKN45 cells and KATO III cells (1 × 104/ well) were lysed and stained by adding hypotonic propidium iodide (50 μg/ml in 0.1% sodium citrate and 0.5% Nonidet P40). After being stained, cells were analysed on a FACScan flow cytometer (EPICS XL; Coulter, Miami, Florida, USA). For both MKN45 and KATO III cells, 10 000 cells were collected. Data were analysed as two dimensional frequency contour plots of forward scatter (linear scale) against propidium iodide stained DNA content (logarithmic scale).
POLYMERASE CHAIN REACTION (PCR) PRIMERS FOR TNF-Rs
Oligonucleotide primers were synthesised using a DNA synthesiser (Gibco BRL Life Technologies, Rockville, Maryland, USA) and were designed from the published sequences.29 ,30 The forward (sense) and reverse (antisense) primers for TNF-RI were 5’-GTGCTGTTGCCCC- TGGTCAT-3’ and 5’-TTCTGCAGCTCCA- GCCG-3’ respectively, with the PCR product being 543 bp. The forward and reverse primers for TNF-RII were 5’-CTCACTTGCCTGC- CGATAAGG-3’ and 5’-TCCCAGCATCAG- GCACTCCAA-3’ respectively, with the PCR product being 450 bp. Forward (sense) and reverse (antisense) primers for β-actin were 5’-CGGAACCGCTCATTGCC-3’ and 5’-ACCCACACTGTGCCCATCTA-3’, yielding a 292 bp product.
DETECTION OF TNF-RI AND TNF-RII mRNA BY REVERSE TRANSCRIPTASE PCR
To determine whether TNF-RI and TNF-RII mRNA were expressed in MKN45 and KATO III cells with and without TNF (100 ng/ml) treatment for 96 hours, we extracted total cellular RNA from freshly isolated cells and performed reverse transcriptase PCR analysis with TNF-RI or TNF-RII specific primers. RNA extraction was performed as follows.29-33 Cells were lysed in Trisol (Gibco BRL Life Technologies), and, after chloroform extraction, RNA was precipitated with propan-2-ol/ethanol and dissolved in diethyl pyrocarbonate-treated water. Then 7 μl of RNA solution was mixed with 1 μl 0.5 mg/ml oligo(dT) and 4 μl of 5 × buffer (250 mM Tris/HCl, pH 8.3, 375 mM KCl) for two minutes at 95°C. After the RNA solution had been left to stand at 37°C for 30 minutes, it was mixed with 2 μl 0.1 M dithiothreitol, 4 μl 2.5 mM dNTPs (Takara Shuzo Co, Kyoto, Japan), 1 μl 10 U/μl RNase inhibitor (Gibco BRL Life Technologies) and 1 μl of 200 U/μl Moloney murine leukaemia virus reverse transcriptase. After reaction at 37°C for 60 minutes, the tube was kept at 90°C for five minutes, followed by chilling on ice. The cDNA was then amplified in a mixture consisting of 2 μl 0.63 U/μl recombinant Taq DNA polymerase (Takara Shuzo Co), 2 μl 2.5 mM dNTPs, 20 μM forward and reverse primers diluted to the appropriate concentration in distilled water (0.2 μM for both TNF-RI and TNF-RII), 2.5 μl 10 × PCR buffer (100 mM Tris/HCl, pH 8.3, 500 mM KCl, 15 mM MgCl2), and distilled water. The PCR was performed in an automated thermocycler (Takara Shuzo Co) at 94°C for four minutes and then 30 cycles of the sequence TNF-RI (one minute at 94°C, 1.5 minutes at 65°C, and three minutes at 72°C) or TNF-RII (one minute at 94°C, two minutes at 60°C, and three minutes at 72°C). Then 10 μl of the PCR product was electrophoresed on 2% agarose gel and visualised by ethidium bromide staining. β-Actin was used as the internal standard.
IMMUNOBLOTTING
To investigate whether or not membrane associated TNF-RI and TNF-RII were expressed by MKN45 and KATO III cells or were changed by TNF (100 ng/ml) treatment for 96 hours, we extracted proteins from the cells and performed western blot analysis. Samples (5 μg/lane) were electrophoresed on 10% sodium dodecyl sulphate polyacrylamide gel under reducing conditions. After electrophoresis, the proteins were transferred electrophoretically to an Immobilon membrane (Millipore, Bedford, Massachusetts, USA). After being blocked, the membrane was incubated for one hour with a mouse polyclonal antibody against human TNF-RI (Genzyme Corp, Boston, Massachusetts, USA) and a goat polyclonal antibody against human TNF-RII (Santa Cruz Biotechnology, Santa Cruz, California, USA). The membrane was washed three times for 15 minutes each with TBS-T (150 mM NaCl, 0.1% NaN3, 50 mM Tris/HCl, pH 7.6, 0.2% Tween 20), and then incubated for one hour with peroxidase conjugated goat anti-mouse IgG (Amersham Pharmacia Biotech UK Ltd, Amersham, Bucks, UK) and rabbit anti-goat IgG (Santa Cruz Biotechnology). After a wash with TBS-T, the membrane was incubated with enhanced chemiluminescence/western blot detecting reagent (Amersham Corp, Arlington Heights, Illinois, USA).
STATISTICAL ANALYSIS
Differences in TNF and sTNF-R production betweenH pylori infected and non-infected subjects were calculated using the Mann-Whitney U test. Correlations between TNF and sTNF-Rs were calculated using Spearman’s rank correlation coefficients. The relation between histology and TNF or sTNF-Rs was analysed using the Kruskal-Wallis test plus one factor analysis of variance or Scheffe’s F test. Statistical analysis of the effect of TNF on MKN45 cell viability was performed using two factor analysis of variance plus Student’s t test. Apoptosis by flow cytometric analysis was analysed using χ2 test. Other data on MKN45 cells and KATO III cells were analysed using Student’s t test. The results were expressed as mean (SD). p<0.05 was considered significant.
Results
TNF AND sTNF-R LEVELS IN TISSUE CULTURE
Production of TNF and sTNF-RII was significantly greater inH pylori positive subjects (n = 34) than inH pylori negative subjects (n = 6) (p<0.01 for both TNF and sTNF-RII) (fig 1A,C). Production of sTNF-RI was also higher in H pylori positive subjects than inH pylori negative subjects, but the difference was not significant (fig1B).
Concentrations of tumour necrosis factor (TNF) and soluble TNF receptors (sTNF-Rs) in tissue culture supernatants of antral mucosa from patients with and without H pylori infection. (A) TNF; (B) sTNF-RI; (C) sTNF-RII. Bars represent mean values. TNF and sTNF-RII levels increased significantly in H pylori positive subjects (**p<0.01).
There was a significant correlation between the TNF and sTNF-RI levels (fig 2A) as well as TNF and sTNF-RII levels (fig 2B) in the medium of tissue cultures from H pylori positive patients (p<0.05 and p<0.01 respectively).
Relation between tumour necrosis factor (TNF) and soluble TNF receptor (sTNF-R) concentrations in tissue culture supernatants from H pylori positive subjects. (A) sTNF-RI, p<0.05, r = 0.344; (B) sTNF-RII, p<0.01, r = 0.523.
Moreover, the TNF level correlated significantly with the inflammation score (p<0.05) (fig 3A). However, no significant correlation was found between sTNF-R levels and the inflammation score (fig 3B,C). The activity, atrophy, and metaplasia scores did not correlate with either TNF or sTNF-R levels (data not shown).
Comparison of tumour necrosis factor (TNF) and soluble TNF receptor (sTNF-R) expression according to inflammation score. (A) TNF, *p<0.05; (B) sTNF-RI; (C) sTNF-RII. Bars represent mean values.
EFFECT OF TNF ON MKN45 CELL VIABILITY
After incubation of MKN45 cells with TNF for 96 hours, cell viability was slightly decreased and this was not dependent on the dose (fig 4).
Effect of tumour necrosis factor (TNF) on MKN45 cell viability. Data are expressed as mean (SD) (n = 8). *p<0.05. There are significant differences (p<0.05) between control and 1 ng/ml and 100 ng/ml TNF.
EFFECT OF TNF ON sTNF-R RELEASE FROM GASTRIC EPITHELIAL CELLS
In contrast, TNF caused dose dependent sTNF-RI release after culture with MKN45 cells for 24 hours (fig 5A) (the maximal response was 188.159 (17.772) pg/ml protein) (p<0.01). sTNF-RII was also released from MKN45 cells and maximal stimulation was observed with 10 ng/ml TNF (46.765 (3.932) pg/ml protein) (p<0.05) (fig 5B). sTNF-RI (fig 5C) and sTNF-RII (fig 5D) were also released from KATO III cells after culture with 100 ng/ml TNF.
Release of soluble tumour necrosis factor (TNF) receptors (sTNF-Rs) from gastric epithelial cells by various concentrations of TNF for 24 hours. (A) sTNF-RI from MKN45 cells; (B) sTNF-RII from MKN45 cells; (C) sTNF-RI from KATO III cells; (D) sTNF-RII from KATO III cells. Data are expressed as mean (SD) (n = 8). *p<0.05, **p<0.01 v control. †p<0.01.
TARGET OF ANTI-sTNF-R MONOCLONAL ANTIBODIES
Anti-sTNF-R monoclonal antibodies bind to shed TNF-Rs, but not to membrane bound TNF-Rs (fig 6A,B).
Target of anti-soluble tumour necrosis factor receptor (sTNF-R) monoclonal antibodies. (A) Anti-sTNF-RI; (B) anti-sTNF-RII. Lane 1, MKN45 cell lysate; lane 2, MKN45 cell culture supernatant.
EFFECT OF ANTI-sTNF-R MONOCLONAL ANTIBODIES ON MKN45 CELL VIABILITY
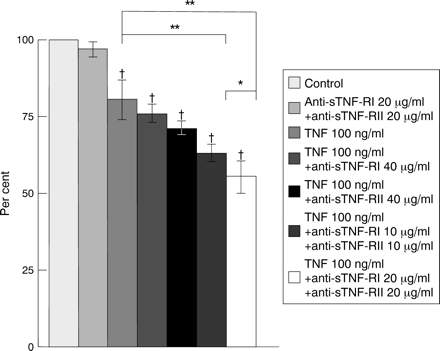
As shown in fig 7, cells cultured with either TNF + anti-sTNF-RI monoclonal antibody (40 μg/ml) or TNF + anti-sTNF-RII monoclonal antibody (40 μg/ml) showed a slight decrease in MTT reduction to about 95% and 88% respectively of that for cells cultured with TNF alone. Furthermore, combined treatment with TNF and both monoclonal antibodies (10 or 20 μg/ml) significantly reduced cell viability to 78% and 69% of that for TNF alone at 10 and 20 μg/ml respectively (p<0.01 v TNF alone). Reduction of cell viability was dependent on the concentrations of both anti-sTNF-R monoclonal antibodies (p<0.05). However, treatment with monoclonal antibodies alone or with control goat IgG (40 μg/ml) did not alter cell viability (data not shown).
Influence of anti-soluble tumour necrosis factor (TNF) receptor I (sTNF-RI) monoclonal antibody and/or anti-sTNF-RII monoclonal antibody on TNF cytotoxicity. The reduction in cell viability was dependent on the concentrations of both types of anti-sTNF-R monoclonal antibody. Data are expressed as mean (SD) (n = 6). †p<0.01 v control. *p<0.05; **p<0.01.
DNA ELECTROPHORESIS
This reduction in cell viability induced by treatment with TNF and anti-sTNF-RI monoclonal antibody and anti-sTNF-RII monoclonal antibody was ascribed to apoptotic DNA fragmentation (DNA ladder), although TNF alone showed a weak DNA ladder (fig 8).
Agarose gel electrophoresis of DNA extracted from MKN45 cells exposed to tumour necrosis factor (TNF) and anti-soluble TNF receptor (sTNF-R) monoclonal antibodies for 96 hours. Lane 1, control (no TNF or anti-sTNF-R monoclonal antibodies); lane 2, TNF 100 ng/ml; lane 3, TNF 100 ng/ml + anti-sTNF-RI and anti-sTNF-RII monoclonal antibodies 20 μg/ml. Addition of anti-sTNF-R monoclonal antibodies induced a more conspicuous DNA ladder.
DETECTION OF APOPTOSIS BY FLOW CYTOMETRIC ANALYSIS
Three cell subpopulations were identified. We have designated these subpopulations area 1 (high DNA content), 2 (low DNA content and markedly decreased forward scatter), and 3 (low DNA content and increased forward scatter). We consider that area 1 contains normal diploid cells. Apoptotic cells were recognised in the low DNA content area (hypodiploid cell population),28 and cell debris was recognised appropriately raising the forward scatter threshold,26 so we consider that area 2 contains debris and area 3 contains apoptotic cells. In MKN45 and KATO III control groups, the percentage of apoptosis was 3.0. We found a much higher percentage of MKN45 cells showing apoptosis with anti-sTNF-R monoclonal antibody treatment (9.6) than the percentage of the same cells treated with TNF alone (6.2) (p<0.01) (fig 9A). Moreover, these effects were stronger on KATO III cells than on MKN45 cells and the percentage of apoptosis with anti-sTNF-R monoclonal antibody treatment was greater than that with only TNF treatment (20.2 and 5.9 respectively) (p<0.01) (fig 9B).
Detection of apoptosis by flow cytometric analysis. (A) MKN45 cells. (a) Cells were cultured for 96 hours in control medium; (b) cells were treated with tumour necrosis factor (TNF) 100 ng/ml alone; (c) cells were treated with TNF 100 ng/ml + anti-soluble TNF receptor I (sTNF-RI) and anti-sTNF-RII monoclonal antibodies 20 μg/ml. (B) KATO III cells. (a)–(c) are the same as for MKN45 cells. These are representative of two dimensional frequency contour plots of forward scatter (linear scale) v propidium iodide (PI)-DNA content (logarithmic scale). Apoptosis was recognised in area 3. (A) (a), (b), (c): 3.0%, 6.2%, 9.6% respectively. (B) (a), (b), (c): 3.0%, 5.9%, 20.2% respectively. Addition of anti-sTNF-R monoclonal antibodies induced apoptosis more conspicuously (p<0.01).
EXPRESSION OF mRNA FOR TNF-RI AND TNF-RII
Figure 10A shows that the TNF-RI specific primers amplified a 543 bp fragment from TNF treated and untreated MKN45 cells. This was consistent with the fragment size predicted from the TNF-RI sequence. Similar results were obtained for TNF-RII (fig 10B), with the predicted 450 bp fragment being found in TNF treated and untreated MKN45 cells. These results suggest that mRNA for both TNF-RI and TNF-RII were expressed equally by MKN45 cells with or without TNF treatment. In further experiments, we obtained similar results for TNF-RI (fig 10C) and TNF-RII (fig 10D) in TNF treated and untreated KATO III cells. These results show that mRNA for both TNF-RI and TNF-RII treated with TNF were expressed slightly more strongly than that without treatment.
Detection of mRNA for tumour necrosis factor receptor I (TNF-RI) and TNF-RII in gastric epithelial cells by reverse transcriptase polymerase chain reaction (PCR). Ethidium bromide stained gels containing PCR products of reverse transcribed TNF-RI mRNA (A) and TNF-RII mRNA (B) in MKN45 cells, and TNF-RI mRNA (C) and TNF-RII mRNA (D) in KATO III cells. Lane 1, 100 bp DNA markers; lane 2, mRNA from untreated cells; lane 3, mRNA from cells treated with TNF 100 ng/ml for 96 hours. In (A) and (B), mRNA of lanes 2 and 3 were expressed equally. In (C) and (D), mRNA of lane 3 was expressed slightly more strongly than that of lane 2.
EXPRESSION OF MEMBRANE ASSOCIATED TNF-RI AND TNF-RII
In contrast, fig 11A shows a decrease in 55 kDa TNF-RI and fig 11B shows a decrease in 75 kDa TNF-RII expression also in MKN45 cells after addition of TNF for 96 hours. Similar results were observed in experiments using KATO III cells (fig11C,D).
Immunoblotting analysis of membrane associated tumour necrosis factor (TNF) receptor I (TNF-RI) and TNF-RII on gastric epithelial cells with and without TNF (100 ng/ml) for 96 hours. (A) 55 kDa TNF-RI in MKN45 cells; (B) 75 kDa TNF-RII in MKN45 cells; (C) 55 kDa TNF-RI in KATO III cells; (D) 75 kDa TNF-RII in KATO III cells. Lane 1, untreated; lane 2, treated with TNF. Expression of TNF-RI and TNF-RII decreased after treatment with TNF.
Discussion
Recently, soluble receptors have been the subject of much attention. For example, there are reports that animals with septicaemia can be protected from shock by administration of recombinant soluble receptor.19 In the clinical realm, application of recombinant gene products including TNF receptors and interleukin 4 receptors is being investigated. However, there is still insufficient understanding of the relation between TNF and sTNF-Rs in gastric mucosa.
This investigation shows that the TNF level correlates significantly with the inflammation score but not with the activity score, suggesting that TNF may be released from inflammatory cells, mainly mononuclear cells. On the other hand, the sTNF-R levels did not correlate with the inflammation and activity scores but correlated significantly with TNF level. This suggests that sTNF-Rs may be released by cells within the gastric mucosa, such as epithelial cells, rather than by inflammatory cells. We selected asymptomatic subjects in order to obtain unbiased results. Because of the predominance of men in the Japanese workforce, 90% of the subjects in this study were male.
It has been reported that normal gastric chief cells express the TNF-Rs and that TNF induces cytotoxicity in these cells.34 Thus we focused on gastric epithelial cells, and investigated the effect of TNF on the epithelium, and the role of sTNF-Rs in counteracting that effect by experiments using MKN45 cells. In some experiments, KATO III cells were also used for comparative studies. In this study, we used a gastric epithelial cancer cell line, because we could not obtain cultured normal gastric epithelial cells established for experimentation. We think that release of sTNF-Rs and cytotoxicity induced by TNF are not only characteristics of cancer cells but also of normal gastric epithelial cells.34 In the presence of TNF, sTNF-R release from MKN45 and KATO III cells increased, and cell damage induced by TNF was increased by sTNF-R neutralising antibodies, suggesting that the effect of TNF may be regulated through sTNF-R release from gastric epithelial cells. Furthermore, the TNF-R response to TNF was not related to a change at the mRNA level in MKN45 cells, but rather to a decrease at the protein level. From these results, we were able to confirm that TNF-R expression was downregulated. If we assume that TNF-R expression was downregulated, it is reasonable to assume that a decrease in MKN45 cell viability was not dependent on TNF concentration (fig 4). Receptor downregulation may be one mechanism of protecting against the harmful effects of TNF. Downregulation of the TNF-Rs has been reported previously.35 ,36 We showed that TNF induced apoptosis was regulated by both increased sTNF-R release from gastric epithelial cells and decreased membrane surface TNF-R expression on these cells. Indeed, the host defence mechanism is thought to involve shedding of the extracellular domain of the receptor, which not only reduces the number of binding sites at the cell surface, but also increases binding protein for circulating TNF and thus prevents attachment to cell surface receptors.
Differences in the actions of TNF-RI and TNF-RII on the membrane surface have already been reported.15 However, there have been no reports on differences in the actions of sTNF-RI and sTNF-RII. Our investigations were also unable to clarify differences in the functions of the two sTNF-Rs. In this study, neutralisation of either sTNF-RI or sTNF-RII did not reduce cell viability very much but neutralisation of both significantly reduced cell viability (fig 7). These results suggest that each sTNF-R may have the ability to block the effect of TNF.
The action of TNF on each cell type present in gastric mucosa of patients infected with H pylori is not yet clear. However, as has been variously reported, it is thought that TNF is able to induce apoptosis in gastric epithelial cells.9 ,10 We confirmed weak apoptosis by DNA laddering and flow cytometric analysis. It has been reported that apoptosis in gastric epithelial cells triggers glandular atrophy, reducing secretion of acid and pepsin, and thus is connected with carcinogenesis.7 It is also said that increased apoptosis may be the stimulus for a compensatory hyperproliferative and potentially preneoplastic response in chronic H pylori infection.5 We surmise that gastric epithelial cells release sTNF-Rs as a protection against these dangers, thereby controlling the action of TNF. However, it is conjectured that, when the balance between TNF and sTNF-Rs is upset, the action of TNF becomes predominant and causes cell damage, beginning with apoptosis.
In conclusion, this study suggests that TNF is produced after infection by H pylori, and this is accompanied by sTNF-R release from gastric epithelial cells. Moreover, release of sTNF-Rs from gastric epithelial cells and apoptosis induced by anti-sTNF-R monoclonal antibodies combined with TNF suggest that the soluble receptors regulate the action of TNF. In addition, TNF-R expression was confirmed to be downregulated. However, elucidation of the mechanism involved requires further research.
Acknowledgments
This work is supported by a Monbusho international scientific research program and a grant from the Ministry of Education, Science, and Culture, Japan. We would like to thank to Mr M Bodman, language consultant of our department, for suggestions on language and style.
Abbreviations used in this paper
- TNF
- tumour necrosis factor
- sTNF-R
- soluble TNF receptor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PCR
- polymerase chain reaction
References
Linked Articles
- Commentary