Abstract
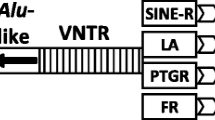
The complement component C4 genes of Old World primates exhibit a long/short dichotomous size variation, except that chimpanzee and gorilla only contain short C4 genes. In human it has been shown that the long C4 gene is attributed to the integration of an endogenous retrovirus, HERV-K(C4), into intron 9. This 6.36 kilobase retroviral element is absent in short C4 genes. Here it is shown that the homologous endogenous retrovirus, ERV-K(C4), is present precisely at the same position in the long C4 gene of orangutan and African green monkey. Determination of the short C4 gene intron 9 sequences from human, three apes, two Old World monkeys, and a New World monkey allowed the establishment of consistent phylogenetic trees for primates, which favors a chimpanzee-gorilla clade. The 5′ long terminal repeats (LTR) and 3′ LTR of ERV-K(C4) in long C4 genes of human, orangutan, and African green monkey have similar sequence divergence values of 9.1%–10.5%. These values are more than five-fold higher than the sequence divergence of the homologous intron 9 sequences between the long and short C4 genes in higher primates. The latter is probably a result of homogenization or concerted evolution. We suggest that the 5′ LTR and 3′ LTR of an endogenous retrovirus can serve as a reliable reference point or a molecular clock for studies of gene duplication and gene evolution. This is because the 5′/3′ LTR sequences were identical at the time of retroviral integration and evolved independently of each other afterwards. Our data provides strong evidence for the short C4 gene being the ancestral form in primates, trans-species evolution, and the “slow-down” phenomenon of the sequence divergence in great apes.
Similar content being viewed by others
References
Andrews, P. Evolution and environment in the Hominoidea. Nature 360: 641–646, 1992
Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. and Struhl, K. Current Protocols in Molecular Biology, Greene Publishing Associates and Wiley-Interscience, New York, 1987
Avise, J. C. Interpretive Tools. In J. C. Avise (ed.): Molecular Markers, Natural History and Evolution, pp. 92–138, Chapman and Hall, New York, 1994
Bontrop, R. E., Broos, L. A. M., Otting, N., and Jonker, M. J. Polymorphism of C4 and CYP21 genes in various primate species. Tissue Antigens 37: 145–151, 1991
Bristow, J., Tee, M. K., Gitelman, S. E., Mellon, S. H., and Miller, W. L. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol 122: 265–278, 1993
Britten, R. J. Rates of DNA sequence evolution differ between taxonomic groups. Science 231: 1393–1398, 1986
Carroll, M. C., Belt, T., Palsdottir, A., and Porter, R. R. Structure and organization of the C4 genes. Philos Trans R Soc Lond 306: 379–388, 1984
Dangel, A. W., Mendoza, A. R., Baker, B. J., Daniel, C. M., Carroll, M. C., Wu, L-C., and Yu, C. Y. The dichotomous size variation of human complement C4 gene is mediated by a novel family of endogenous retroviruses which also establishes species-specific genomic patterns among Old World primates. Immunogenetics 40: 425–436, 1994
Felsenstein, J. Phylip Manual, version 3.4, Berkley Herbarium, University of Berkley, Berkley, 1991
Fitch, W. M. and Margolish, E. Toward defining the course of evolution: minimum change for a specific tree topology. Sys Zool 20: 406–416, 1971
Genetics Computer Group, Inc. GCG Sequence Analysis Software Package: Program Manual, Version 7, Genetics Computer Group, Inc., Madison, 1991
Granados, J., Aweh, Z. L., Chen, J.-H., Giles, C. M., Balmer, H., Yunis, E. J., and Alper, C. A. There are two C4 genetic loci and a null allele in the chimpanzee. Immunogenetics 26: 344–350, 1987
Green, H. and Djian, P. Consecutive actions of different gene-altering mechanisms in the evolution of involucrin. Mol Biol Evol 9: 977–1017, 1992
Higgins, D. G. and Sharp, P. M. Fast and sensitive multiple sequence alignment on microcomputer. CABIOS 5: 151–153, 1989
Horai, S., Satta, Y., Hayasaka, K., Kondo, R., Inoue, T., Ishida, T., Hayashi, S., and Takahata, N. Man's place in Hominoidea revealed by mitochondrial DNA genealogy. J Mol Evol 35: 32–43, 1992
Kawaguchi, H. and Klein, J. Organization of C4 and CYP21 loci in gorilla and orangutan. Hum Immunol 33: 153–162, 1992
Kawaguchi, H., O'hUigin, C., and Klein, J. Evolution of primate C4 and CYP21 genes. In J. Klein and D. Klein (eds.): Molecular Evolution of the Major Histocompatibility Complex, pp. 357–381, Springer, Heidelberg, 1991
Kawaguchi, H., O'hUigin, C., and Klein, J. Evolutionary origin of mutations in the primate cytochrome P450c21 gene. Am J Hum Genet 50: 766–780, 1992a
Kawaguchi, H., Zaleska-Rutczynska, Z., Figueroa, F., O'hUigin, C., and Klein, J. C4 genes of the chimpanzee, gorilla, and orang-utan: evidence for extensive homogenization. Immunogenetics 35: 16–23, 1992b
Kawamura, S., Saitou, N., and Ueda, S. Concerted evolution of the primate immunoglobulin α-gene through gene conversion. J Biochem 267: 7359–7367, 1992
Klein, J., Takahata, N., and Ayala, F. J. MHC polymorphism and human origins. Sci Am 269: 78–83, 1993
Kluge, A. G. and Farris, J. S. Quantitative phylogenetics and the evolution of anurans. Sys Zool 18: 1–32, 1969
Li, W.-H. and Tanimura, M. The molecular clock runs more slowly in man than in apes and monkeys. Nature 326: 93–96, 1987
Mariani-Costantini, R., Horn, T. M., and Callahan, R. Ancestry of a human endogenous retrovirus family. J Virol 63: 4982–4985, 1989
Mayer, W. E., Jonker, M., Klein, D., Ivanyi, P., van Severter, G., and Klein, J. Nucleotide sequence of chimpanzee MHC class I alleles: evidence for trans-species mode of evolution. EMBO J 7(9): 2765–2774, 1988
Minghetti, P. P. and Dugaiczyk, A. The emergence of new DNA repeats and divergence of primates. Proc Natl Acad Sci USA 90: 1872–1876, 1993
Miyamoto, M. M., Slightom, J. L., and Goodman, M. Phylogenetic relations of humans and African apes from DNA sequences in the psi eta-globin region. Science 238: 369–373, 1987
Ono, M., Yasunaga, T., Miyata, T., and Ushikubo, H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol 60: 589–598, 1986
Paz-Artal, E., Corell, A., Alvarez, M., Varela, P., Allende, L., Mardrono, A., Rosal, M., and Arnaiz-Villena, A. C4 gene polymorphism in primates: evolution, generation, and Chido and Rodgers antigenicity. Immunogenetics 40: 381–396, 1994
Porter, R. R. Complement polymorphism, the major histocompatibility complex and associated diseases: a speculation. Mol Biol Med 1: 161–168, 1983
Ruvolo, M., Disotell, T. R., Allard, M. W., Brown, W. M., and Honeycutt, R. L. Resolution of the African hominoid trichotomy by use of a mitochondrial gene sequence. Proc Natl Acad Sci USA 88: 1570–1574, 1991
Saiki, R. Amplification of genomic DNA.M.A. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (eds.): PCR Protocols: A Guide to Methods and Applications, pp. 13–20. Academic Press, San Diego, 1990
Saitou, N. and Nei, M. The neighbour-joining method: a new method for reconstructing evolutionary trees. Mol Biol Evol 4: 406–425, 1987
Shen, L. M., Wu, L. C., Sanlioglu, S., Chen, R., Mendoza, A. R., Dangel, A., Carroll, M. C., Zipf, W., and Yu, C. Y. Structure and genetics of the partially duplicated gene RP located immediately upstream of the complement C4A and C4B genes in the HLA class II region: molecular cloning, exon-intron structure, composite retroposon and breakpoint of gene duplication. J Biol Chem 269: 8466–8476, 1994
Sneath, P. H. A. and Sokal, R. R. Numerical Taxonomy, W. H. Freeman and Co., San Francisco, 1973
Wilkinson, D. A., Mager, D. L., and Leong, J. C. Endogenous Human Retroviruses. In J. A. Levy (ed.): The Retroviridae (vol 3), pp. 465–535. Plenum Press, New York, 1994
Yu, C. Y. The complete exon-intron structure of a human complement component C4A gene: DNA sequences, polymorphism, and linkage to the 21-hydroxylase genes. J Immunol 146: 1057–1066, 1991
Yu, C. Y., Belt, K. T., Giles, C. M., Campbell, R. D., and Porter, R. R. Structural basis of the polymorphism of human complement component C4A and C4B: gene size, reactivity and antigenicity. EMBO J 5: 2873–2881, 1986
Yu, C. Y. and Campbell, R. D. Definitive RFLPs to distinguish between the human complement C4A/C4B isotypes and the major Rodgers/Chido determinants: application to the study of C4 null alleles. Immunogenetics 25: 384–390, 1987
Zhang, W. J., Christiansen, F. T., Wu, X., Abraham, L. J., Giphart, M., and Dawkins, R. L. Organization and evolution of C4 and CYP21 genes in primates: importance of genomic segments. Immunogenetics 37: 170–176, 1993
Zhu, Z.-B., Jian, B., and Volanakis, J. E. Ancestry of SINE-R.C2: a human specific retroposon. Hum Genet 93: 545–551, 1994
Author information
Authors and Affiliations
Additional information
The nucleotide sequence data reported in this paper have been submitted to the GenBank nucleotide sequence database and have been assigned the accession numbers L38796-L38807
Rights and permissions
About this article
Cite this article
Dangel, A.W., Baker, B.J., Mendoza, A.R. et al. Complement component C4 gene intron 9 as a phylogenetic marker for primates: long terminal repeats of the endogenous retrovirus ERV-K(C4) are a molecular clock of evolution. Immunogenetics 42, 41–52 (1995). https://doi.org/10.1007/BF00164986
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00164986